Time is Limited - Act Now

Phillips® CPAP and Ventilation Machines are Causing Damage That is Being Reported
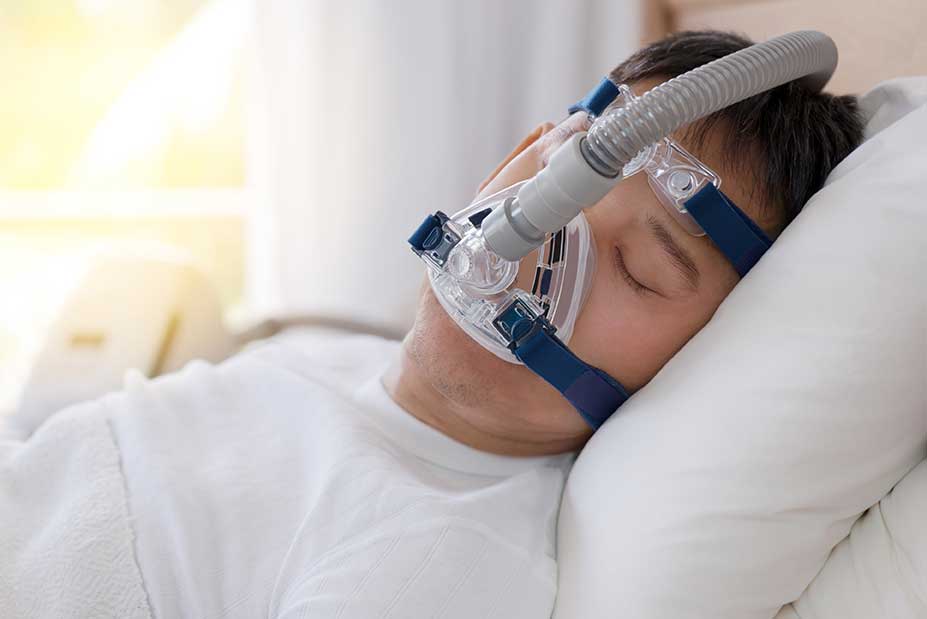
Philips Respironics® recalled millions of CPAP and BiPAP machines because degraded PE-PUR sound abatement foam in the machines may break down and cause serious health issues, including:
- Cancer
- Respiratory inflammation and
- Other toxic effects
The Philips® CPAP recall includes the Philips Dreamstation and other popular CPAP machines.
Phillips® has announced that they’re recalling between 3 and 4 million CPAP and ventilation devices due to disintegrating sound-reducing foam.
Consumers have begun to report suffering serious side effects that include kidney/liver damage, wide ranging chemical poisonings, and other unhealthy conditions.
Many consumers experience serious and long-lasting side effects from the chemical from the recalled machines, including cancers, strokes, and a variety of cancers.
Consumers that have used particular Phillips® CPAP and ventilation machines and suffered any of these symptoms should come forward to file claims for compensation — legal case evaluations are free of charge and private.
Which Philips© CPAP Ventilators and Machines Have Been Recalled?

With a surge in the number of Philips© CPAP ventilators and machines linked to respiratory issues, chemical poisonings, and much more, recalls on these pieces of medical equipment are more frequent, as well.
The recall doesn’t affect all of Philips®’ breathing devices.
About 80% of the affected devices are CPAP machines and about 20% are ventilators, Philips® spokesman Steve Klink told Reuters.
Most of the devices come from Philips’ DreamStation® line.
The recall affects all serial numbers of the following affected devices manufactured between 2009 and April 26, 2021.
More than half of the affected devices are in the U.S., according to Philips®.
In January 2022, the FDA announced that Trilogy Evo® ventilators and Trilogy Evo® repair kits not originally recalled in the July 2021 recall were added to the recalled devices list.
Recalled Philips® device brands include:
- Aeris®
- BiPAP A30/A40 Series Device Models®
- BiPAP V30®
- C-Series ASV®
- Dorma 400 and 500®
- DreamStation®
- E30®
- Garbin Plus®
- LifeVent®
- REMstar SE Auto®
- SystemOne (Q-Series)®
- Trilogy 100®
- Trilogy 200®
- Trilogy Evo®
Those patients who have been injured by Philips© CPAP ventilators and machines are encouraged to come forward for a free, private case evaluation that could lead the way to legal compensation.
Our network of attorneys have a track record of success.
IS THERE A CASE?
If anyone suffers from CPAP ventilators, there may be compensation awarded.
EXPERIENCED LAWYERS
Our experts have extensive experience in CPAP machine litigation and are ready to fight in court.
GET STARTED NOW
Contact us right away to set up a free and confidential case review.
Please seek the advice of a medical professional before making health care decisions. This advertisement is not associated with Philips® or any government agency.
www.submitcases.com is the property of Shield Legal LLC. 5170 Badura Ave Las Vegas, NV 89118
This website is not part of the Facebook website or Facebook, Inc. Additionally, this site is NOT endorsed by Facebook in any way. FACEBOOK is a trademark of FACEBOOK, INC.
ATTORNEY ADVERTISING. This Website is not intended to provide medical advice. Consult your doctor or physician before starting or stopping any medication.
Discontinuing a prescribed medication without your doctor’s advice can result in injury or death. are not an indication of future results. Every case is evaluated on its own facts and circumstances. Valuation depends on facts, injuries, jurisdiction, venue, witnesses, parties, and testimony, among other factors. No representation is made that the quality of legal services to be performed is greater than the quality of legal services performed by other lawyers. Submit Cases does not itself provide legal services. Cases will be referred to third party attorneys and law firms. Do not rely on this advertisement in making any medical decision. Please call your physician before making any medical decision, including altering your use of any drug. Court costs and case expenses may be the responsibility of the client. Not available in all states. This advertisement is not intended as a testimonial, endorsement or dramatization, and does not constitute a guarantee, warranty, or prediction regarding the outcome of your legal matter, either expressed or implied. Anyone considering a lawyer should independently investigate the lawyers' credentials and ability, and not rely upon advertisements or self-proclaimed expertise. Only persons age 18 or older have permission to access our Service. Our Service does not address anyone under the age of 13("Children").
Privacy Policy | Terms and Conditions | CCPA Privacy Notice | Do Not Sell My Info
©2024 Submit Cases. All Rights Reserved